Nanomotors as probes to sense cancer environment
Author: Admin; Date: 21/04/2022
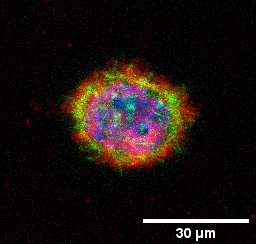
Cofounders of Theranautilus along with an interdisciplinary team of researchers from the Indian Institute of Science (IISc) has used a 3-D tumor model and magnetically driven nanomotors to probe the microenvironment of cancer cells. The team consists of researchers from the Center for Nano Science and Engineering (CeNSE) and Department of Molecular Reproduction, Development and Genetics (MRDG). In their work, published in Angewandte Chemie, the team steered helical nanomotors remotely via an external magnetic field through the tumor model to sense, map and quantify changes in the cellular environment. The model comprises both healthy and cancer cells embedded within a reconstituted basement membrane matrix, and mimics the breast cancer environment.
The study highlights a new way of targeting cancer cells by manoeuvering nanomotors inside a tumor and waiting for them to localize in the vicinity of the cancerous site. “We tried driving the nanomotors toward cancer cells in a tumor model and observed them getting stuck to the matrix near cancer cells, but this was not observed near normal cells,” says Dr. Debayan Dasgupta, a co-first author and CEO, Theranautilus. The extracellular matrix (ECM) is a complex 3-D network of proteins and carbohydrates secreted by living cells into their neighborhood. However, when cancer cells secrete fresh material into the ECM, it disrupts the chemical and physical composition of the native ECM surrounding healthy cells, degrading the local environment.
Therefore, understanding how the cellular microenvironment is altered due to cancer cells and measuring these changes quantitatively could be vital in understanding the progression of cancer. In the current study, the researchers discovered that as the nanomotors approached the cancer cell membrane, they stuck to the matrix more strongly than they would to normal cells. To measure how strongly the nanomotors bound to the matrix, the team calculated the magnetic field strength required to overcome the adhesive force, and move forward.
“This means that the cancer cells are doing something. So, we did some measurements and discovered that it [the adhesive force] depended on the type of cells, the strength of interaction and also which side of the cell the nanomotor approached,” explains Ambarish Ghosh, Professor at CeNSE and Director, Theranautilus. “In the end, we really ended up discovering a physical property of an important biological environment.”
“What came as a beautiful surprise was that within such a milieu, we found that aggressive cancer cells ended up remodeling their surroundings by making them stickier, and richer in specific charged sugars,” says Ramray Bhat, Assistant Professor at MRDG and one of the senior authors. “This charging can potentially be used to target and kill tiny populations of cancer cells hidden among their normal counterparts, for which we are extending these studies to living animals.”
Mobile Nanobots for Prevention of Root Canal Treatment Failure
Author: Admin; Date: 08/06/2022
The use of magnetic field-controlled nanobots at the nano-scale can effectively eliminate bacteria deep within dentinal tubules, enhancing the success of root canal treatments. These procedures aim to treat tooth infections by removing infected tissue and flushing the tooth with antibiotics or chemicals. However, the current methods often fail to completely eradicate all bacteria, particularly antibiotic-resistant strains like Enterococcus faecalis, which remain concealed within microscopic canals called dentinal tubules. In a recent study published in Advanced Healthcare Materials, researchers developed helical nanobots comprised of silicon dioxide coated with iron. These nanobots could be manipulated using a device generating a low-intensity magnetic field. When injected into extracted tooth samples, the nanobots’ movement was tracked under a microscope. By adjusting the magnetic field frequency, the researchers were able to control the nanobots’ precise motion and facilitate their penetration into the dentinal tubules. Importantly, the team could induce heat generation on the nanobots’ surface using the magnetic field, which effectively killed bacteria in close proximity. This method surpasses previous techniques like ultrasound or laser pulses, which can only penetrate a maximum distance of 800 micrometers before their energy dissipates. The nanobots, on the other hand, demonstrated penetration capabilities of up to 2,000 micrometers. The use of heat for bacterial elimination also offers a safer alternative to harsh chemicals or antibiotics. The researchers have conducted successful and safe tests of the dental nanobots in mice models. Additionally, they are currently developing a compact medical device that can be easily inserted into the mouth, enabling dentists to inject and manipulate the nanobots during root canal treatments. Future research will focus on regulatory and clinical trials.

